Corporate Member Update- Genentech
Corporate Member Update- Genentech
TECENTRIQ HYBREZA (atezolizumab/hyaluronidase-tqjs)
November, 2024
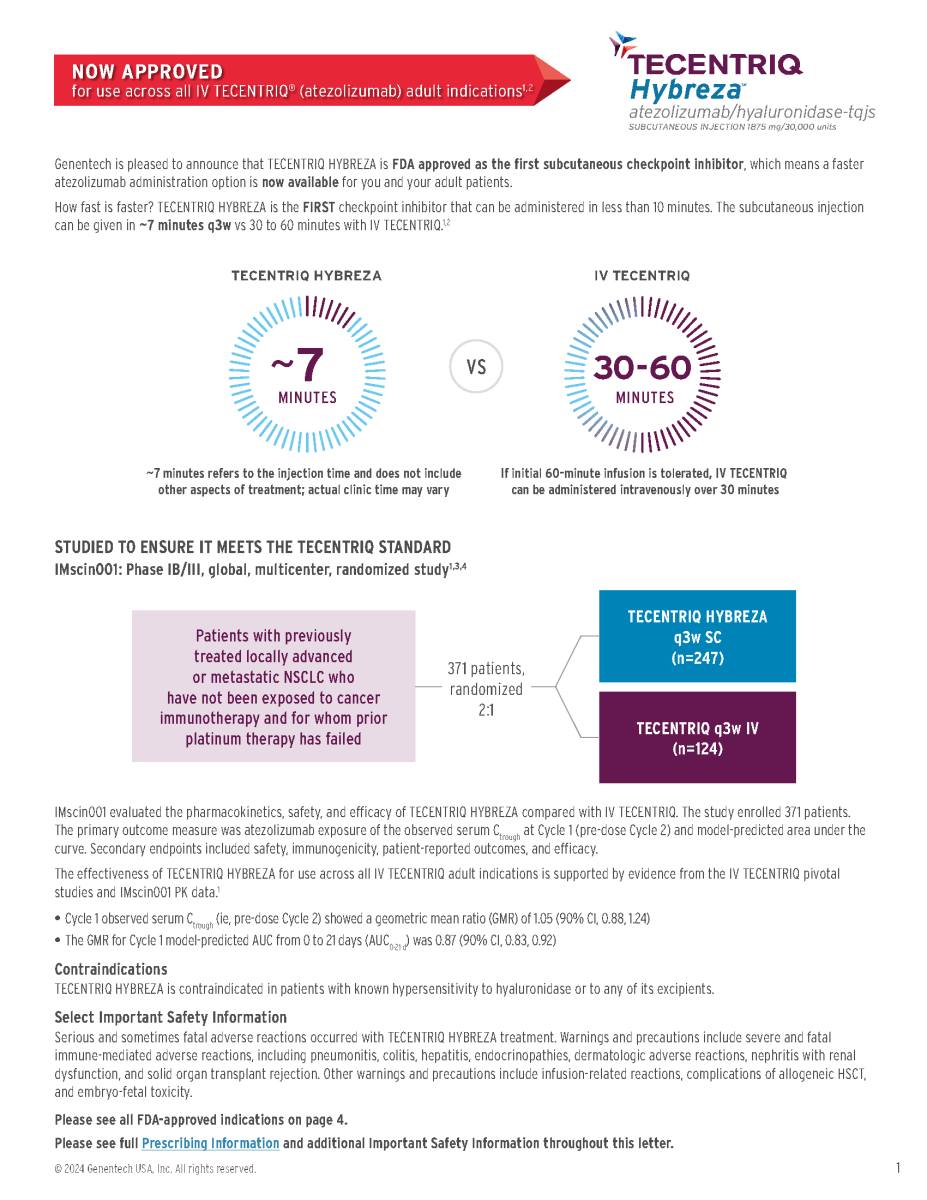
Genentech is pleased to announce that TECENTRIQ HYBREZA is FDA approved as the first subcutaneous checkpoint inhibitor, which means a faster
atezolizumab administration option is now available for you and your adult patients.
For the full press release, please click here.