Latest News/Therapies
Latest News/Therapies
Below you will find information and links to new cancer therapies or recent FDA changes of approvals of medication offered by NOS Corporate Members! Drugs and Products will stay online for 12 months from approval date.
Corporate Member Update: Merck
Approval of KEYTRUDA QLEXTM (pembrolizumab and berahyaluronidase alfa-pmph)
KEYTRUDA QLEXTM (pembrolizumab and berahyaluronidase alfa-pmph) Subcutaneous Injection 165 mg/2,000 units per mL is indicated for use in adult patients across most solid tumor indications for KEYTRUDA® (pembrolizumab) Injection 100 mg—whether alone or in combination with other therapies.
• One such indication is the adjuvant treatment of adult patients with renal cell carcinoma (RCC) at intermediate-high or high risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions.
To read the full memo, please click here.
Corporate Member Update: Amgen
IMPORTANT UPDATE: Full FDA Approval Granted in 2L ES-SCLC
IMDELLTRA® (tarlatamab-dlle) is now fully FDA-approved for the treatment of adult patients with extensive stage small cell lung cancer (ES-SCLC) with disease progression on or after platinum-based chemotherapy. IMDELLTRA is the first and only DLL3-targeting Bispecific T-cell Engager (BiTE®) therapy approved in this setting, representing an important milestone for patients and providers.
This approval was based on results from the Phase 3 DeLLphi-304 trial, which was a phase 3, multicenter, randomized, open-label trial evaluating the efficacy and safety of IMDELLTRA® compared with chemotherapy in 509 patients with ES-SCLC who had progressed after treatment with 1 prior line of platinum-containing chemotherapy, with or without a PD-(L)1 inhibitor. Patients were randomized 1:1 to receive IMDELLTRA® (n=254) or chemotherapy (n=255).
The study demonstrated a median OS of 13.6 months with IMDELLTRA® compared to 8.3 months with chemotherapy, with a 40% reduction in the risk of death (HR 0.60; p<0.001).1,2
The most common (> 20%) adverse reactions in clinical trials of IMDELLTRA(R) were CRS (57%), fatigue (48%), decreased appetite (38%), dysgeusia (34%), pyrexia (33%), constipation (31%), musculoskeletal pain (31%), and nausea (25%).2
Please see the Indication and Important Safety Information below, including the Boxed Warnings, as well as the full Prescribing Information.
You can learn more at IMDELLTRAhcp.com.
Click here to read the full memo.
FDA Approves Zepzelca® (lurbinectedin) and Atezolizumab (Tecentriq®) Combination as First-Line Maintenance Therapy for Extensive-Stage Small Cell Lung Cancer
FDA Approves Zepzelca® (lurbinectedin) and Atezolizumab (Tecentriq®) Combination as First-Line Maintenance Therapy for Extensive-Stage Small Cell Lung Cancer
Combination reduced the risk of disease progression or death by 46% and risk of death by 27% in pivotal Phase 3 IMforte trial.
Zepzelca and atezolizumab combination added to National Comprehensive Cancer Network® Guidelines for SCLC.
To read the entire memo, please click here.
AstraZeneca- Datroway J-Code
Information available listed below as well as information on the new Datroway J-Code:
We now have a Pre-approval Information Exchange (PIE) presentation available for SERENA-6 our Phase III randomized trial to assess switching from AI + CDK4/6 inhibitor to camizestrant + CDK4/6 inhibitor in patients with HR+/HER2- locally advanced and metastatic breast cancer with emergent ESR1 mutations. Camizestrant is an investigational product for this trial.
The most recent PIE presentation is for Serena-6 and below you will find the additional PIE presentations that you may be interested in. If there is interest, I can have them covered in one call or we could schedule individual calls (30 min in length). One item that I could use some clarification on is whether you have had our clinical reviews for your teams. With so many approvals in the past years, many accounts have requested clinical reviews which provides a pipeline presentation for our Oncology portfolio. The focus is typically tailored to provide a high-level overview and then streamlined to what is expected to be approved in the next 6-9 months. 2026 will bring more anticipated growth for our franchises with an expected 16 approvals.
Additional PIE presentations available:
MATTERHORN Trial: A phase III global study of perioperative treatment for resectable gastric and gastroesophageal adenocarcinomas using durvalumab with FLOT chemotherapy. Durvalumab combined with FLOT is investigational for these indications.
AMPLIFY Trial: A randomized, multicenter, open-label, phase III study in adult patients with previously untreated CLL to compare the efficacy and safety of acalabrutinib in combination with venetoclax, with and without obinutuzumab, compared to investigator’s choice of chemoimmunotherapy.
Destiny Breast-09 Trial: Fam-Trastuzumab Deruxtecan-nxki is being investigated for the treatment of adult patients with unresectable or metastatic Her2+ breast cancer in the first line setting in combination with pertuzumab, who have not received a piror anti-HER2 regimen for metastatic disease.
Corporate Member Update: Amgen-New Technology Add On Payment/NTAP in SCLC
IMDELLTRA® (tarlatamab-dlle) was granted New Technology Add On Payment (NTAP) by CMS, effective October 1st, 2025 for Medicare Beneficiaries in the inpatient setting.2
To read through full details on the IMDELLTRA® NTAP you can download the attached IMDELLTRA® NTAP Flashcard.
INDICATION
IMDELLTRA® (tarlatamab-dlle) is indicated for the treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC) with disease progression on or after platinum-based chemotherapy.
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
IMPORTANT SAFETY INFORMATION
WARNING: CYTOKINE RELEASE SYNDROME AND NEUROLOGIC TOXICITY including
IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
Cytokine release syndrome (CRS), including serious or life-threatening reactions, can occur in patients receiving IMDELLTRA®. Initiate treatment with IMDELLTRA® using the step-up dosing schedule to reduce the incidence and severity of CRS. Withhold IMDELLTRA® until CRS resolves or permanently discontinue based on severity.
Neurologic toxicity, including immune effector cell-associated neurotoxicity syndrome (ICANS), including serious or life-threatening reactions, can occur in patients receiving IMDELLTRA®. Monitor patients for signs and symptoms of neurologic toxicity, including ICANS, during treatment and treat promptly. Withhold IMDELLTRA® until ICANS resolves or permanently discontinue based on severity.
WARNINGS AND PRECAUTIONS
Cytokine Release Syndrome (CRS): IMDELLTRA® can cause CRS including serious or life-threatening reactions. In the pooled safety population, CRS occurred in 55% of patients who received IMDELLTRA®, including 34% Grade 1, 19% Grade 2, 1.1% Grade 3 and 0.5% Grade 4. Recurrent CRS occurred in 24% of patients, including 18% Grade 1 and 6% Grade 2.
Most events (43%) of CRS occurred after the first dose, with 29% of patients experiencing any grade CRS after the second dose and 9% of patients experiencing CRS following the third dose or later. Following the Day 1, Day 8, and Day 15 infusions, 16%, 4.3% and 2.1% of patients experienced ≥ Grade 2 CRS, respectively. The median time to onset of all grade CRS from most recent dose of IMDELLTRA® was 13.5 hours (range: 1 to
268 hours). The median time to onset of ≥ Grade 2 CRS from most recent dose of IMDELLTRA® was 14.6 hours (range: 2 to 566 hours).
Clinical signs and symptoms of CRS included pyrexia, hypotension, fatigue, tachycardia, headache, hypoxia, nausea, and vomiting. Potentially life-threatening complications of CRS may include cardiac dysfunction, acute respiratory distress syndrome, neurologic toxicity, renal and/or hepatic failure, and disseminated intravascular coagulation (DIC).
Administer IMDELLTRA® following the recommended step-up dosing and administer concomitant medications before and after Cycle 1 IMDELLTRA® infusions as described in Table 3 of the Prescribing Information (PI) to reduce the risk of CRS. Administer IMDELLTRA® in an appropriate health care facility equipped to monitor and manage CRS. Ensure patients are well hydrated prior to administration of IMDELLTRA®.
Closely monitor patients for signs and symptoms of CRS during treatment with IMDELLTRA®. At the first sign of CRS, immediately discontinue IMDELLTRA® infusion, evaluate the patient for hospitalization and institute supportive care based on severity. Withhold or permanently discontinue IMDELLTRA® based on severity. Counsel patients to seek medical attention should signs or symptoms of CRS occur.
Neurologic Toxicity, Including ICANS: IMDELLTRA® can cause serious or life-threatening neurologic toxicity, including ICANS. In the pooled safety population, neurologic toxicity, including ICANS, occurred in 47% of patients who received IMDELLTRA®, including 10% Grade 3. The most frequent neurologic toxicities were headache (14%), peripheral neuropathy (7%), dizziness (7%), insomnia (6%), muscular weakness (3.7%), delirium (2.1%), syncope (1.6%), and neurotoxicity (1.1%).
ICANS occurred in 9% of IMDELLTRA®-treated patients. Recurrent ICANS occurred in 1.6% of patients. Most patients experienced ICANS following Cycle 2 Day 1 (24%). Following Day 1, Day 8, and Day 15 infusions, 0.5%, 0.5% and 3.7% of patients experienced ≥ Grade 2 ICANS, respectively. The median time to onset of ICANS from the first dose of IMDELLTRA® was 29.5 days (range: 1 to 154 days). ICANS can occur several weeks following administration of IMDELLTRA®. The median time to resolution of ICANS was 33 days (range: 1 to 93 days).
The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS. Clinical signs and symptoms of ICANS may include but are not limited to confusional state, depressed level of consciousness, disorientation, somnolence, lethargy, and bradyphrenia.
Patients receiving IMDELLTRA® are at risk of neurologic adverse reactions and ICANS resulting in depressed level of consciousness. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, in the event of any neurologic symptoms until they resolve.
Closely monitor patients for signs and symptoms of neurologic toxicity and ICANS during treatment. At the first sign of ICANS, immediately evaluate the patient and provide supportive therapy based on severity. Withhold IMDELLTRA® or permanently discontinue based on severity.
Cytopenias: IMDELLTRA® can cause cytopenias including neutropenia, thrombocytopenia, and anemia. In the pooled safety population, decreased neutrophils occurred in 12% including 6% Grade 3 or 4 of IMDELLTRA®-treated patients. The median time to onset for Grade 3 or 4 neutropenia was 29.5 days (range: 2 to 213). Decreased platelets occurred in 33% including 3.2% Grade 3 or 4. The median time to onset for Grade 3 or 4 decreased platelets was 50 days (range: 3 to 420). Decreased hemoglobin occurred in 58% including 5% Grade 3 or 4. Febrile neutropenia occurred in 0.5% of patients treated with IMDELLTRA®.
Monitor patients for signs and symptoms of cytopenias. Perform complete blood counts prior to treatment with IMDELLTRA®, before each dose, and as clinically indicated. Based on the severity of cytopenias, temporarily withhold, or permanently discontinue IMDELLTRA®.
Infections: IMDELLTRA® can cause serious infections, including life-threatening and fatal infections.
In the pooled safety population, infections, including opportunistic infections, occurred in 41% of patients who received IMDELLTRA®. Grade 3 or 4 infections occurred in 13% of patients. The most frequent infections were COVID-19 (9%, majority during the COVID-19 pandemic), urinary tract infection (10%), pneumonia (9%), respiratory tract infection (3.2%), and candida infection (3.2%).
Monitor patients for signs and symptoms of infection prior to and during treatment with
IMDELLTRA® and treat as clinically indicated. Withhold or permanently discontinue IMDELLTRA® based on severity.
Hepatotoxicity: IMDELLTRA® can cause hepatotoxicity. In the pooled safety population, elevated ALT occurred in 42%, with Grade 3 or 4 ALT elevation occurring in 2.1%. Elevated AST occurred in 44% of patients, with Grade 3 or 4 AST elevation occurring in 3.2%. Elevated bilirubin occurred in 15% of patients; Grade 3 or 4 total bilirubin elevations occurred in 1.6% of patients. Liver enzyme elevation can occur with or without concurrent CRS. Monitor liver enzymes and bilirubin prior to treatment with IMDELLTRA®, before each dose, and as clinically indicated. Withhold IMDELLTRA® or permanently discontinue based on severity.
Hypersensitivity: IMDELLTRA® can cause severe hypersensitivity reactions. Clinical signs and symptoms of hypersensitivity may include, but are not limited to, rash and bronchospasm. Monitor patients for signs and symptoms of hypersensitivity during treatment with IMDELLTRA® and manage as clinically indicated. Withhold or consider permanent discontinuation of IMDELLTRA® based on severity.
Embryo-Fetal Toxicity: Based on its mechanism of action, IMDELLTRA® may cause fetal harm when administered to a pregnant woman. Advise patients of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with IMDELLTRA® and for 2 months after the last dose.
ADVERSE REACTIONS
The most common (> 20%) adverse reactions were CRS (55%), fatigue (51%), pyrexia (36%), dysgeusia (36%), decreased appetite (34%), musculoskeletal pain (30%), constipation (30%), anemia (27%), and nausea (22%). The most common (≥ 2%) Grade 3 or 4 laboratory abnormalities were decreased lymphocytes (57%), decreased sodium (16%), increased uric acid (10%), decreased total neutrophils (6%), decreased hemoglobin (5%), increased activated partial thromboplastin time (5%), decreased potassium (5%), increased aspartate aminotransferase (3.2%), decreased white blood cells (3.8%), decreased platelets (3.2%), and increased alanine aminotransferase (2.1%).
Serious adverse reactions occurred in 58% of patients. Serious adverse reactions in > 3% of patients included CRS (24%), pneumonia (6%), pyrexia (3.7%), and hyponatremia (3.6%). Fatal adverse reactions occurred in 2.7% of patients including pneumonia (0.5%), aspiration (0.5%), pulmonary embolism (0.5%), respiratory acidosis (0.5%), and respiratory failure (0.5%).
DOSAGE AND ADMINISTRATION: Important Dosing Information
Administer IMDELLTRA® as an intravenous infusion over one hour.
Administer IMDELLTRA® according to the step-up dosing schedule in the IMDELLTRA® PI (Table 1) to reduce the incidence and severity of CRS.
For Cycle 1, administer recommended concomitant medications before and after Cycle 1 IMDELLTRA® infusions to reduce the risk of CRS reactions as described in the PI (Table 3).
IMDELLTRA® should only be administered by a qualified healthcare professional with appropriate medical support to manage severe reactions such as CRS and neurologic toxicity including ICANS.
Due to the risk of CRS and neurologic toxicity, including ICANS, monitor patients from the start of the IMDELLTRA® infusion for 22 to 24 hours on Cycle 1 Day 1 and Cycle 1 Day 8 in an appropriate healthcare setting.
Recommend that patients remain within 1 hour of an appropriate healthcare setting for a total of 48 hours from start of the infusion with IMDELLTRA® following Cycle 1 Day 1 and Cycle 1 Day 8 doses, accompanied by a caregiver.
Prior to administration of IMDELLTRA®, evaluate complete blood count, liver enzymes, and bilirubin before each dose, and as clinically indicated.
Ensure patients are well hydrated prior to administration of IMDELLTRA®.
Please see IMDELLTRA® full Prescribing Information, including BOXED WARNINGS.
Corporate Member Update: Merck
Merck would like to inform you that KEYTRUDA® (pembrolizumab) Injection 100 mg is approved for 42 indications across 18 types of cancer.
• One indication that the FDA has now approved is KEYTRUDA for the treatment of adult patients with resectable locally advanced head and neck squamous cell carcinoma (HNSCC) whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-approved test, as a single agent as neoadjuvant treatment, continued as adjuvant treatment in combination with radiotherapy (RT) with or without cisplatin and then as a single agent.
Click here to read the full press release!
Corporate Member Update- Jazz Pharmaceuticals
Jazz Pharmaceuticals Announces U.S. FDA Approval of Ziihera® (zanidatamab-hrii) for the Treatment of Adults with Previously Treated, Unresectable or Metastatic HER2-positive (IHC 3+) Biliary Tract Cancer (BTC).
Read the full press release here.
Corporate Member Update- Amgen
We’re excited to share that LUMAKRAS® (sotorasib) in combination with Vectibix® (panitumumab) is approved in a new indication. Please see the product website and the full Prescribing Information for LUMAKRAS® and Vectibix® for more information including the BOXED WARNING and Important Safety Information.
Corporate Member Update- BeiGene now BeOne Medicines
BeiGene Unveils Proposed Name Change to BeOne Medicines, Reaffirming Its Mission to Unite Global Community Against Cancer
New name reflects Company’s bold vision to eradicate cancer by harnessing the transformative power of global collaboration and multisectoral partnerships
Company will change its Nasdaq ticker to ONC
SAN MATEO, Calif. & BASEL, Switzerland--(BUSINESS WIRE)-- BeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global oncology company, today announced its intent to change the Company’s name to BeOne Medicines Ltd., confirming its commitment to develop innovative medicines to eliminate cancer by partnering with the global community to serve as many patients as possible.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241114671168/en/
Corporate Member Update-BeiGene
TEVIMBRA added to NCCN Guidelines for Esophageal and Esophagogastric Junction Cancers and Gastric Cancer.
Objective
To inform Solid Tumor team of recent updates to the National Comprehensive Cancer Network® (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Esophageal and Esophagogastric Junction Cancers (Version 1.2025) and Gastric Cancer (Version 1.2025).
To read the whole memo, please click here.
Corporate Member Update- Bristol Myers Squibb
U.S. Food and Drug Administration Approves Opdivo® (nivolumab) plus Yervoy® (ipilimumab) as a First-Line Treatment for Unresectable or Metastatic Hepatocellular Carcinoma
Based on the Phase 3 CheckMate-9DW trial, Opdivo plus Yervoy demonstrated a statistically significant overall survival benefit compared to investigator’s choice of lenvatinib or sorafenib1
In the trial, 38% of patients were still alive at 3 years with this dual immunotherapy vs. 24% with the comparator arm1
To see the full press release, please click here.
Corporate Member Update- Amgen
New Coding Guidance for Amgen Product
IMDELLTRA® (tarlatamab-dlle) received a permanent J-Code J9026, Injection, tarlatamab-dlle, 1 mg
Effective for dates of service on or after January 1, 2025, the Centers for Medicare, and Medicaid Services (CMS) has assigned the following Healthcare Common Procedure Coding Systems (HCPCS) J-Code for IMDELLTRA® J9026, Injection, tarlatamab-dlle, 1 mg
Please see full prescribing information, including BOXED Warnings.
https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Imdelltra/imdelltra_fpi.pdf
Corporate Member Update- AstraZeneca
IMFINZI
Now available for the treatment of adult patients with LS-SCLC whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
To download the full new indication document, please click here.
IMFINZI EPIC® EHR System Guide
Tipsheet for Order Sets, Patient Lists, and EHR Alerts
To download the full EHR System Guide document, please click here.
For more information, please visit www.imfinzihcp.com.
The US Food and Drug Administration (FDA) has approved a new indication for IMFINZI® (durvalumab) for the treatment of adult patients with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT). Approval was based on results from the Phase III, global, double-blind, placebo-controlled ADRIATIC trial.
Please feel free to reach out with any questions or to request a presentation.
IMPORTANT SAFETY INFORMATION
There are no contraindications for IMFINZI® (durvalumab).
Immune-Mediated Adverse Reactions
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate. Withhold or permanently discontinue IMFINZI depending on severity. See USPI Dosing and Administration for specific details. In general, if IMFINZI requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Immune-Mediated Pneumonitis
IMFINZI can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation. In patients who did not receive recent prior radiation, the incidence of immune-mediated pneumonitis was 2.4% (34/1414), including fatal (<0.1%), and Grade 3-4 (0.4%) adverse reactions. The incidence of pneumonitis (including radiation pneumonitis) in patients with LS-SCLC following chemoradiation within 42 days prior to initiation of IMFINZI in ADRIATIC was 14% (37/262) in patients receiving IMFINZI and 6% (16/265) in patients receiving placebo. Of the patients who received IMFINZI (262), 0.4% had a fatal adverse reaction and 2.7% had Grade 3 adverse reactions.
Immune-Mediated Colitis
IMFINZI can cause immune-mediated colitis that is frequently associated with diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. Immune-mediated colitis occurred in 2% (37/1889) of patients receiving IMFINZI, including Grade 4 (<0.1%) and Grade 3 (0.4%) adverse reactions.
Immune-Mediated Hepatitis
IMFINZI can cause immune-mediated hepatitis. Immune-mediated hepatitis occurred in 2.8% (52/1889) of patients receiving IMFINZI, including fatal (0.2%), Grade 4 (0.3%) and Grade 3 (1.4%) adverse reactions.
Immune-Mediated Endocrinopathies
Adrenal Insufficiency: IMFINZI can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Immune-mediated adrenal insufficiency occurred in 0.5% (9/1889) of patients receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions.
Hypophysitis: IMFINZI can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate symptomatic treatment including hormone replacement as clinically indicated. Grade 3 hypophysitis/hypopituitarism occurred in <0.1% (1/1889) of patients who received IMFINZI.
Thyroid Disorders: IMFINZI can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement therapy for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated.
Thyroiditis: Immune-mediated thyroiditis occurred in 0.5% (9/1889) of patients receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions.
Hyperthyroidism: Immune-mediated hyperthyroidism occurred in 2.1% (39/1889) of patients receiving IMFINZI.
Hypothyroidism: Immune-mediated hypothyroidism occurred in 8.3% (156/1889) of patients receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions.
Type 1 Diabetes Mellitus, which can present with diabetic ketoacidosis: Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Grade 3 immune-mediated Type 1 diabetes mellitus occurred in <0.1% (1/1889) of patients receiving IMFINZI.
Immune-Mediated Nephritis with Renal Dysfunction
IMFINZI can cause immune-mediated nephritis. Immune-mediated nephritis occurred in 0.5% (10/1889) of patients receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions.
Immune-Mediated Dermatology Reactions
IMFINZI can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Immune-mediated rash or dermatitis occurred in 1.8% (34/1889) of patients receiving IMFINZI, including Grade 3 (0.4%) adverse reactions.
Other Immune-Mediated Adverse Reactions
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMFINZI or were reported with the use of other
PD-1/PD-L1 blocking antibodies.
Cardiac/vascular: Myocarditis, pericarditis, vasculitis.
Nervous system: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy.
Ocular: Uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Pancreatitis including increases in serum amylase and lipase levels, gastritis, duodenitis.
Musculoskeletal and connective tissue disorders: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatic.
Endocrine: Hypoparathyroidism.
Other (hematologic/immune): Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenia, solid organ transplant rejection, other transplant (including corneal graft) rejection.
Infusion-Related Reactions
IMFINZI can cause severe or life-threatening infusion-related reactions. Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMFINZI based on the severity. See USPI Dosing and Administration for specific details. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses. Infusion-related reactions occurred in 2.2% (42/1889) of patients receiving IMFINZI, including Grade 3 (0.3%) adverse reactions.
Complications of Allogeneic HSCT after IMFINZI
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on its mechanism of action and data from animal studies, IMFINZI can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. In females of reproductive potential, verify pregnancy status prior to initiating IMFINZI and advise them to use effective contraception during treatment with IMFINZI and for 3 months after the last dose of IMFINZI.
Lactation
There is no information regarding the presence of IMFINZI in human milk; however, because of the potential for adverse reactions in breastfed infants from IMFINZI, advise women not to breastfeed during treatment and for 3 months after the last dose.
Adverse Reactions
· In patients with limited-stage SCLC in the ADRIATIC study receiving IMFINZI (n=262), the most common adverse reactions occurring in ≥20% of patients receiving IMFINZI were pneumonitis or radiation pneumonitis (38%), and fatigue (21%). The most common Grade 3 or 4 adverse reactions (≥3%) were pneumonitis or radiation pneumonitis and pneumonia.
· In patients with limited-stage SCLC in the ADRIATIC study receiving IMFINZI (n=262), IMFINZI was permanently discontinued due to adverse reactions in 16% of the patients receiving IMFINZI. Serious adverse reactions occurred in 30% of patients receiving IMFINZI. The most frequent serious adverse reactions reported in ≥1% of patients receiving IMFINZI were pneumonitis or radiation pneumonitis (12%), and pneumonia (5%). Fatal adverse reactions occurred in 2.7% of patients who received IMFINZI including pneumonia (1.5%), cardiac failure, encephalopathy, and pneumonitis (0.4% each).
The safety and effectiveness of IMFINZI has not been established in pediatric patients.
Indication:
IMFINZI, as a single agent, is indicated for the treatment of adult patients with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
Please see full Prescribing Information including Medication Guide for IMFINZI.
You may report side effects related to AstraZeneca products.
Reference: IMFINZI® (durvalumab) [Prescribing Information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2024.
Corporate Member Update- Menarini Stemline- ELEGANT clinical trial listing MED-05207 11/24
ELEGANT clinical trial listing MED-05207 11/24
A Phase 3 Study of elacestrant versus standard endocrine therapy in node-positive ER+, HER2- early breast cancer with high risk of recurrence (ELEGANT)
Despite advances in the adjuvant treatment of early-stage estrogen receptor-positive (ER+), human epidermal growth factor receptor 2-negative (HER2-) breast cancer, there continues to be a risk of recurrence. The ELEGANT study is a Global, Multicenter, Randomized, Open-label Phase 3 Study with the primary goal to evaluate the efficacy and safety of elacestrant versus standard endocrine therapy in participants with node-positive ER+/HER2- early breast cancer with high risk of recurrence. The main purpose of this study is to compare the Invasive Breast Cancer-Free Survival (IBCFS) over a period of up to 5 years. 4220 participants will be included in this study around the world. Participants will have equal chance to be assigned to receive either of the treatment combinations.
Or email elegantstudy.com and/or click on below QR code

Corporate Member Update- AstraZeneca

The US Food and Drug Administration (FDA) has approved a new indication for IMFINZI® (durvalumab), in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI continued as a single agent as adjuvant treatment after surgery, is indicated for the treatment of adult patients with resectable (tumors ≥4 cm and/or node positive) non-small cell lung cancer (NSCLC) and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements. Approval was based on results from the Phase III, global, double-blind, placebo-controlled AEGEAN trial.
Please see Important Safety Information below.
Indication:
IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI continued as a single agent as adjuvant treatment after surgery, is indicated for the treatment of adult patients with resectable (tumors ≥4 cm and/or node positive) non-small cell lung cancer (NSCLC) and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements.
IMPORTANT SAFETY INFORMATION
There are no contraindications for IMFINZI® (durvalumab).
Immune-Mediated Adverse Reactions
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying
immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate. Withhold or permanently discontinue IMFINZI depending on severity. See USPI Dosing and Administration for specific details. In general, if IMFINZI requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Immune-Mediated Pneumonitis
IMFINZI can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation. In patients who did not receive recent prior radiation, the incidence of immune-mediated pneumonitis was 2.4% (34/1414), including fatal (<0.1%), and Grade 3-4 (0.4%) adverse reactions. In patients who received recent prior radiation, the incidence of pneumonitis (including radiation pneumonitis) in patients with unresectable Stage III NSCLC following definitive chemoradiation within 42 days prior to initiation of IMFINZI in PACIFIC was 18.3% (87/475) in patients receiving IMFINZI and 12.8% (30/234) in patients receiving placebo. Of the patients who received IMFINZI (475), 1.1% were fatal and 2.7% were Grade 3 adverse reactions. The frequency and severity of immune-mediated pneumonitis in patients who did not receive definitive chemoradiation prior to IMFINZI were similar in patients who received IMFINZI as a single agent or with ES-SCLC or BTC when given in combination with chemotherapy.
Immune-Mediated Colitis
IMFINZI can cause immune-mediated colitis that is frequently associated with diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. Immune-mediated colitis occurred in 2% (37/1889) of patients receiving IMFINZI, including Grade 4 (<0.1%) and Grade 3 (0.4%) adverse reactions.
Immune-Mediated Hepatitis
IMFINZI can cause immune-mediated hepatitis. Immune-mediated hepatitis occurred in 2.8% (52/1889) of patients receiving IMFINZI, including fatal (0.2%), Grade 4 (0.3%) and Grade 3 (1.4%) adverse reactions.
Immune-Mediated Endocrinopathies
· Adrenal Insufficiency: IMFINZI can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Immune-mediated adrenal insufficiency occurred in 0.5% (9/1889) of patients receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions.
· Hypophysitis: IMFINZI can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate symptomatic treatment including hormone replacement as clinically indicated. Grade 3 hypophysitis/hypopituitarism occurred in <0.1% (1/1889) of patients who received IMFINZI.
· Thyroid Disorders: IMFINZI can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement therapy for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated.
· Thyroiditis: Immune-mediated thyroiditis occurred in 0.5% (9/1889) of patients receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions.
· Hyperthyroidism: Immune-mediated hyperthyroidism occurred in 2.1% (39/1889) of patients receiving IMFINZI.
· Hypothyroidism: Immune-mediated hypothyroidism occurred in 8.3% (156/1889) of patients receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions.
· Type 1 Diabetes Mellitus, which can present with diabetic ketoacidosis: Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Grade 3 immune-mediated Type 1 diabetes mellitus occurred in <0.1% (1/1889) of patients receiving IMFINZI.
Immune-Mediated Nephritis with Renal Dysfunction
IMFINZI can cause immune-mediated nephritis. Immune-mediated nephritis occurred in 0.5% (10/1889) of patients receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions.
Immune-Mediated Dermatology Reactions
IMFINZI can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including
Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Immune-mediated rash or dermatitis occurred in 1.8% (34/1889) of patients receiving IMFINZI, including Grade 3 (0.4%) adverse reactions.
Other Immune-Mediated Adverse Reactions
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMFINZI or were reported with the use of other
PD-1/PD-L1 blocking antibodies.
· Cardiac/vascular: Myocarditis, pericarditis, vasculitis.
· Nervous system: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy.
· Ocular: Uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
· Gastrointestinal: Pancreatitis including increases in serum amylase and lipase levels, gastritis, duodenitis.
· Musculoskeletal and connective tissue disorders: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatic.
· Endocrine: Hypoparathyroidism.
· Other (hematologic/immune): Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenia, solid organ transplant rejection, other transplant (including corneal graft) rejection.
Infusion-Related Reactions
IMFINZI can cause severe or life-threatening infusion-related reactions. Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMFINZI based on the severity. See USPI Dosing and Administration for specific details. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses. Infusion-related reactions occurred in 2.2% (42/1889) of patients receiving IMFINZI, including Grade 3 (0.3%) adverse reactions.
Complications of Allogeneic HSCT after IMFINZI
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on its mechanism of action and data from animal studies, IMFINZI can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. In females of reproductive potential, verify pregnancy status prior to initiating IMFINZI and advise them to use effective contraception during treatment with IMFINZI and for 3 months after the last dose of IMFINZI.
Lactation
There is no information regarding the presence of IMFINZI in human milk; however, because of the potential for adverse reactions in breastfed infants from IMFINZI, advise women not to breastfeed during treatment and for 3 months after the last dose.
Adverse Reactions
· In patients with resectable NSCLC in the AEGEAN study, the most common adverse reactions (occurring in ≥20% of patients) were anemia, nausea, constipation, fatigue, musculoskeletal pain, and rash.
· In patients with resectable NSCLC in the neoadjuvant phase of the AEGEAN study receiving IMFINZI in combination with platinum-containing chemotherapy (n=401), permanent discontinuation of IMFINZI due to an adverse reaction occurred in 6.7% of patients. Serious adverse reactions occurred in 21% of patients. The most frequent (≥1%) serious adverse reactions were pneumonia (2.7%), anemia (1.5%), myelosuppression (1.5%), vomiting (1.2%), neutropenia (1%), and acute kidney injury (1%). Fatal adverse reactions occurred in 2% of patients, including death due to COVID-19 pneumonia (0.5%), sepsis (0.5%), myocarditis (0.2%), decreased appetite (0.2%), hemoptysis (0.2%), and death not otherwise specified (0.2%). Of the 401 IMFINZI treated patients who received neoadjuvant treatment and 398 placebo-treated patients who received neoadjuvant treatment, 1.7% (n=7) and 1% (n=4), respectively, did not receive surgery due to adverse reactions.
· In patients with resectable NSCLC in the adjuvant Phase of the AEGEAN study receiving IMFINZI as a single agent (n=265), permanent discontinuation of adjuvant IMFINZI due to an adverse reaction occurred in 8% of patients. Serious adverse reactions occurred in 13% of patients. The most frequent serious adverse reactions reported in >1% of patients were pneumonia (1.9%), pneumonitis (1.1%), and COVID-19 (1.1%). Four fatal adverse reactions occurred during the adjuvant phase of the study, including COVID-19 pneumonia, pneumonia aspiration, interstitial lung disease and aortic aneurysm.
The safety and effectiveness of IMFINZI has not been established in pediatric patients.
Please see Full Prescribing Information including Medication Guide for IMFINZI.
You may report side effects related to AstraZeneca products.
For more information, please visit www.imfinzihcp.com
Reference: Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
IMFINZI is a registered trademark of the AstraZeneca group of companies.
©2024 AstraZeneca. All rights reserved. US-86200 Last Updated 8/24
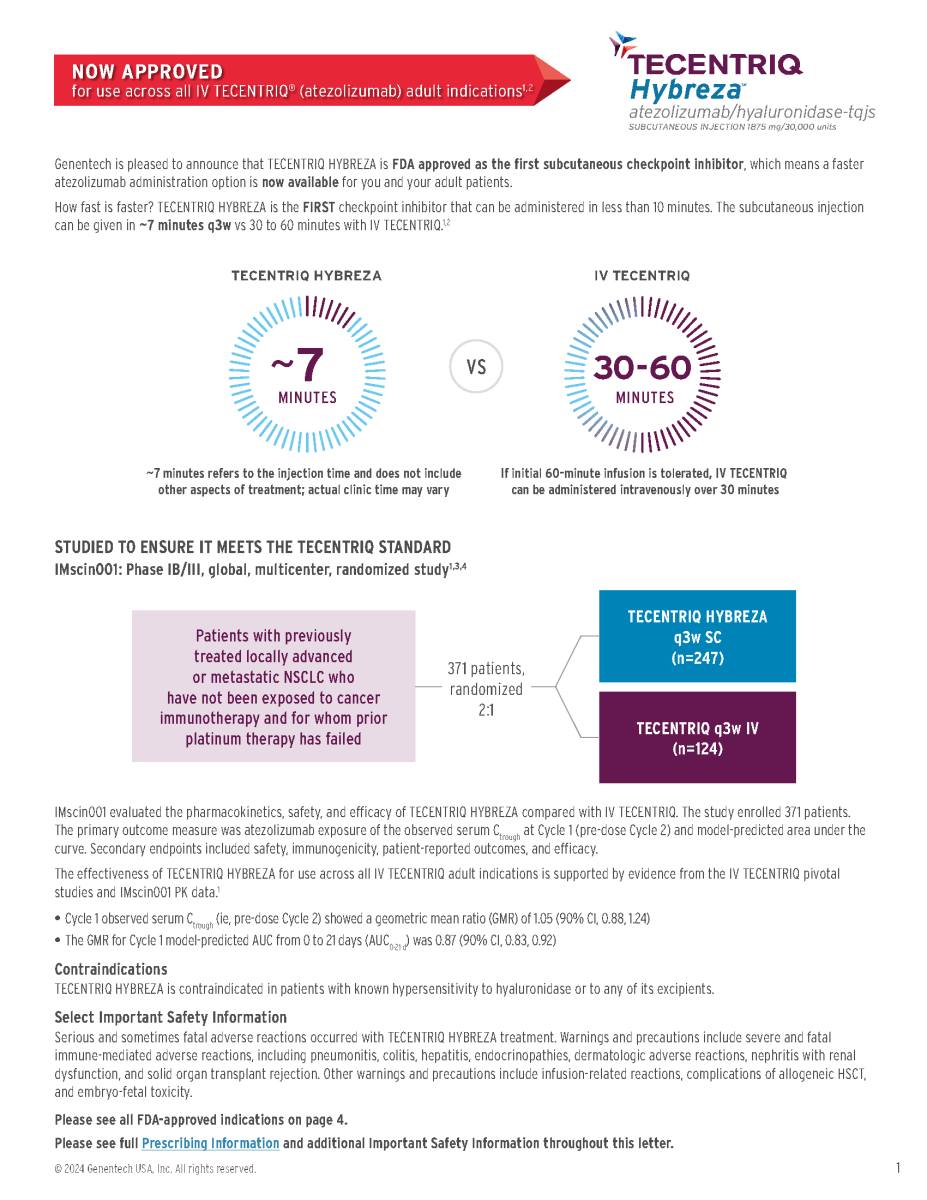
Corporate Member Update- Genentech
Genentech is pleased to announce that TECENTRIQ HYBREZA is FDA approved as the first subcutaneous checkpoint inhibitor, which means a faster
atezolizumab administration option is now available for you and your adult patients.
For the full press release, please click here.
Corporate Member Update- Genentech
NOW APPROVED
- In combination with palbociclib and fulvestrant
- For 1L HR+/HER2- mBC
- With PIK3CA mutation and endocrine resistance
Indication
Itovebi (inavolisib), in combination with palbociclib and fulvestrant, is indicated for the treatment of adults with endocrine-resistant, PIK3CA-mutated, hormone receptor (HR)-positive, human epidermal growth-factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer, as detected by an FDA-approved test, following recurrence on or after completing adjuvant endocrine therapy.
To access the entire packet, please click here!

Corporate Member Update- BeiGene
Please review the update from BeiGene below to announce WAC pricing and that the Centers for Medicare and Medicaid Services (CMS) has assigned a permanent drug-specific J-code TEVIMBRA (tislelizumab-jsgr) injection, for intravenous use, effective October 1, 2024. The J-code (J9329) and WAC are detailed in the attached link below.
Corporate Member Update: Servier
VORANIGO was approved by the FDA to treat mIDH glioma. Please review the information and trial highlights below.
Click here for this update!
Corporate Member Update: Merck New Indication for Pembrolizumab
Treatment for Adult Patients With Primary Advanced or Recurrent Endometrial Carcinoma
KEYTRUDA® (pembrolizumab) Injection 100 mg, in combination with carboplatin and paclitaxel, followed by KEYTRUDA as a single agent, is indicated for the treatment of adult patients with primary advanced or recurrent endometrial carcinoma.
View the complete new indication here.






























